PQQ is more than just a compound; it represents a frontier in nutritional science with the potential to enhance health and longevity. With its unique properties that support mitochondrial function, protect neurons, and promote cardiovascular health, PQQ has piqued the interest of researchers and health enthusiasts alike. As studies continue to explore its benefits, PQQ could become a cornerstone of preventive health strategies, particularly in an aging population facing cognitive decline and metabolic challenges. By unlocking the mysteries of PQQ, we may be able to harness its full potential for improving quality of life and extending healthspan. The journey into understanding PQQ is just beginning, and its implications for wellness are both exciting and promising.
Patients are advised to inform their healthcare providers about their full medical history, including any existing conditions such as bleeding disorders or recent surgeries, as well as any medications they are taking to avoid potential drug interactions. Pregnant or breastfeeding women should also seek medical advice before using pentoxifylline.
Regulatory compliance remains a pivotal aspect of API manufacturing. With increasing scrutiny from regulatory bodies such as the FDA and EMA, manufacturers must adopt rigorous quality control measures to ensure product safety and efficacy. Implementing quality by design (QbD) principles helps manufacturers approach API production systematically, identifying potential risks and establishing controls from the outset.
Once the partially digested carbohydrates reach the small intestine, pancreatic amylase continues the work of salivary amylase. It further breaks down the remaining starches into simple sugars such as glucose, which is a crucial energy source for the body. Without adequate amylase, individuals may experience digestive discomfort and nutrient deficiencies, as carbohydrates are a primary fuel source for the brain and muscles.
Ornithine L-Aspartate is available in various forms, including injectable solutions, oral tablets, and powders. The typical dosage for effective therapeutic use often ranges around 500 mg, depending on the specific condition being addressed and the patient’s medical history. It is essential for users to consult healthcare professionals before starting any new supplement regimen, especially if they have existing health conditions or are taking other medications.
Patients with certain pre-existing conditions should exercise caution when using pentoxifylline. Those with a history of excessive bleeding, heart problems, or recent surgeries may face increased risks. As the drug can affect blood coagulation, it is vital for patients on anticoagulants or blood thinners to confer with their healthcare provider before initiating treatment with pentoxifylline.
Once the API has passed QC and QA checks, it must be submitted for regulatory approval. This involves compiling extensive documentation demonstrating that the API is safe, effective, and manufactured according to the highest standards. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA), review this information before granting approval for the API to be used in drug formulations.


 Disconnect the hose from the pump and remove it from the vehicle Disconnect the hose from the pump and remove it from the vehicle
Disconnect the hose from the pump and remove it from the vehicle Disconnect the hose from the pump and remove it from the vehicle A professional mechanic with experience working on Porsche 928s should perform the replacement to avoid damaging other components in the power steering system A professional mechanic with experience working on Porsche 928s should perform the replacement to avoid damaging other components in the power steering system
A professional mechanic with experience working on Porsche 928s should perform the replacement to avoid damaging other components in the power steering system A professional mechanic with experience working on Porsche 928s should perform the replacement to avoid damaging other components in the power steering system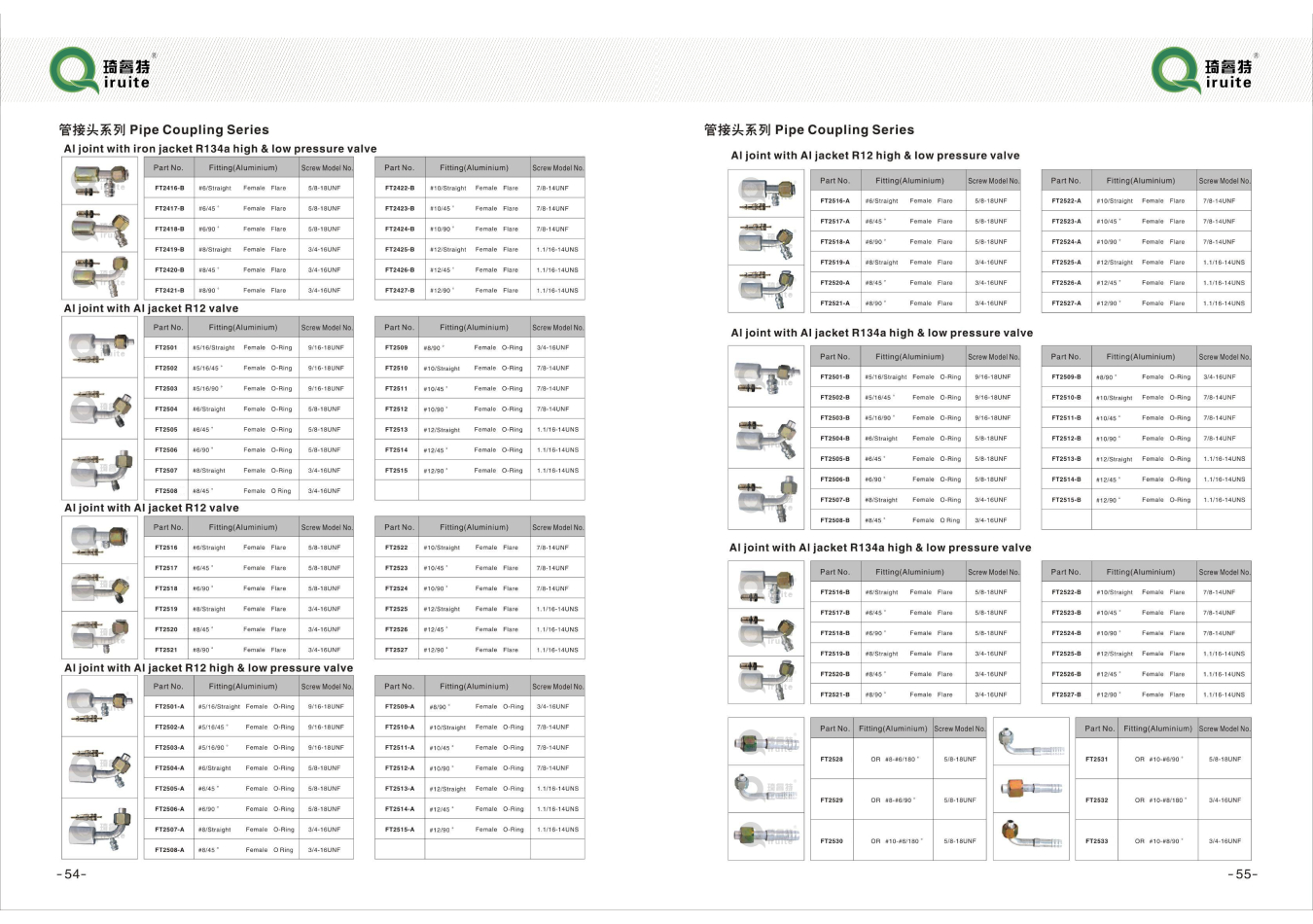
 Secondly, it allows for easy assembly and disassembly, facilitating maintenance and repairs Secondly, it allows for easy assembly and disassembly, facilitating maintenance and repairs
Secondly, it allows for easy assembly and disassembly, facilitating maintenance and repairs Secondly, it allows for easy assembly and disassembly, facilitating maintenance and repairs