pqq secom
-
Regulatory compliance is another significant aspect of the production of APIs. Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe impose stringent guidelines to ensure the quality, safety, and efficacy of pharmaceutical products. Therefore, manufacturers of active pharmaceutical intermediates must adhere to Good Manufacturing Practices (GMP) to maintain compliance. These regulations not only assure the quality of the intermediates but also serve to protect public health by minimizing risks associated with pharmaceutical products.
...
-
3. Flotation This technique is particularly effective in treating wastewater. It uses air bubbles to help lift suspended particles to the surface, where they can be skimmed off. This method is effective for removing fats, oils, grease, and other light particles that may not settle during sedimentation.
...

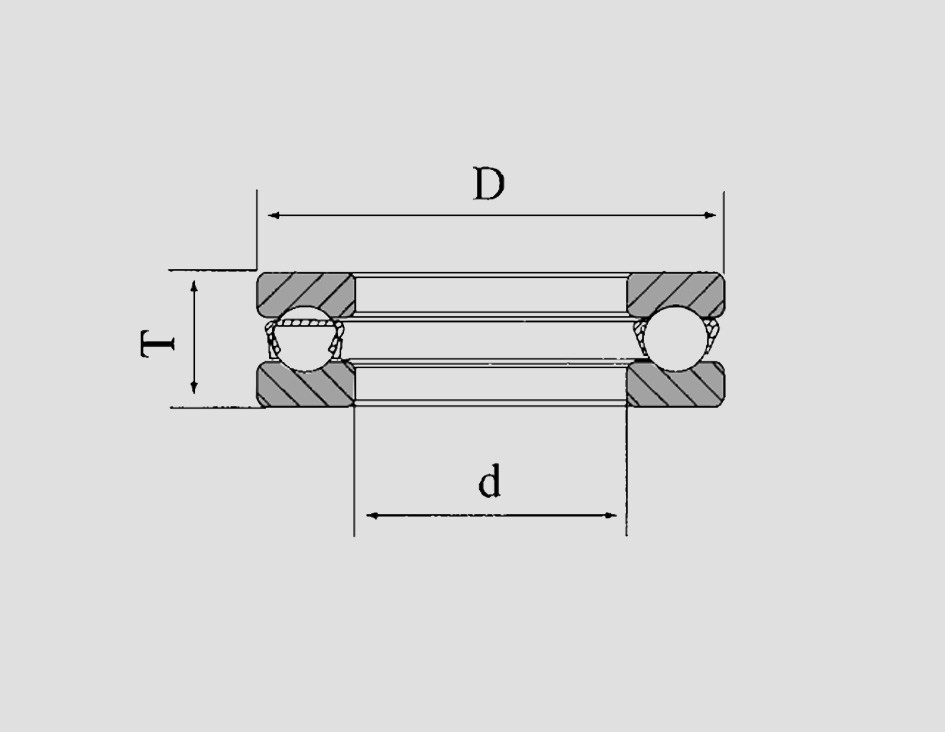 The materials used to manufacture these bearings, such as high-carbon chromium steel, are specifically formulated to withstand harsh operating conditions The materials used to manufacture these bearings, such as high-carbon chromium steel, are specifically formulated to withstand harsh operating conditions
The materials used to manufacture these bearings, such as high-carbon chromium steel, are specifically formulated to withstand harsh operating conditions The materials used to manufacture these bearings, such as high-carbon chromium steel, are specifically formulated to withstand harsh operating conditions
 6 kN, while the basic static load rating (Cor) is 96 kN, while the basic static load rating (Cor) is 9
6 kN, while the basic static load rating (Cor) is 96 kN, while the basic static load rating (Cor) is 9

 For example, in automotive engines, these bearings help to reduce friction and heat generation, leading to improved fuel efficiency and reduced emissions For example, in automotive engines, these bearings help to reduce friction and heat generation, leading to improved fuel efficiency and reduced emissions
For example, in automotive engines, these bearings help to reduce friction and heat generation, leading to improved fuel efficiency and reduced emissions For example, in automotive engines, these bearings help to reduce friction and heat generation, leading to improved fuel efficiency and reduced emissions