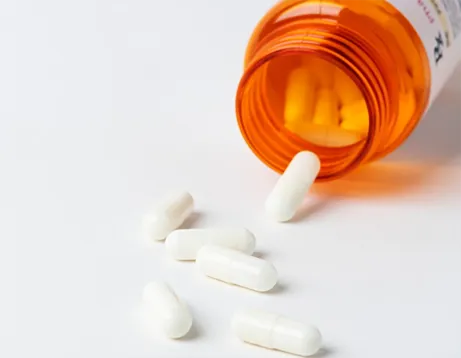
PQQ is known for its role as an antioxidant, protecting cells from oxidative stress and potentially reducing the risk of age-related diseases. In addition to its antioxidant properties, it is believed to support neuroprotection and may play a role in the growth and maintenance of neurons. Users often take Max Q10 Ultra PQQ to help enhance mental clarity, improve memory, and increase overall energy levels, particularly among individuals feeling fatigued or under stress.
Sevoflurane belongs to the class of halogenated ethers and is widely used as an inhalational anesthetic in surgical and medical procedures. Known for its low blood-gas solubility, sevoflurane induces anesthesia quickly while allowing for a rapid emergence from the anesthetic state. These unique properties have contributed to its increasing popularity in various medical settings.
Pharmaceutical intermediates can be classified based on their structure and the type of reactions they undergo. Common categories include amines, esters, ketones, and aldehydes. Each class serves different purposes in pharmaceutical synthesis, often tailored to the specific requirements of the API being manufactured. For instance, certain intermediates may be preferred for the synthesis of antibiotics, while others may be critical in developing anti-cancer drugs.
The use of plastic additives plays a crucial role in the functionality and performance of plastic products. From improving flexibility and durability to enhancing safety and aesthetics, additives contribute to a wide array of properties that meet consumer demands and regulatory requirements. As the industry evolves and faces challenges related to sustainability and health regulations, the development of novel, eco-friendly additives will be essential to ensure the continued viability of plastic materials in our everyday lives. Understanding these additives and their implications is vital for manufacturers, consumers, and policymakers alike in navigating the future of plastics.
Quality control is an integral part of API production. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), impose stringent guidelines to ensure that APIs meet established standards. Compliance with Good Manufacturing Practices (GMP) is mandatory to maintain the quality and consistency of APIs. Furthermore, the analytical techniques employed, such as chromatography and spectroscopy, play a vital role in verifying the identity, strength, and purity of the APIs.
api in drug manufacturing