Once synthesized, intermediates must undergo purification processes, such as crystallization or chromatography, to eliminate impurities and by-products. Quality control is paramount in this industry; every batch of intermediates must be rigorously tested to meet stringent regulatory standards set forth by agencies like the FDA or EMA. This ensures that only high-quality intermediates are used in the production of APIs, safeguarding patient health.
Moreover, sustainability has become a critical factor in procurement strategies. Buyers are increasingly tasked with considering the environmental impact of their sourcing decisions. This includes evaluating suppliers' sustainability practices and looking for ways to minimize waste and reduce carbon footprints throughout the supply chain.
One of the vital aspects of active pharma is its contribution to the development of new and effective treatments. With the increasing prevalence of chronic diseases such as diabetes, cancer, and cardiovascular conditions, the demand for innovative medications has surged. Pharmaceutical companies are continuously investing in research and development (R&D) to discover novel APIs that can address unmet medical needs. This focus on innovation is coupled with advancements in technology, including biotechnology and nanotechnology, which have opened new avenues for drug development.


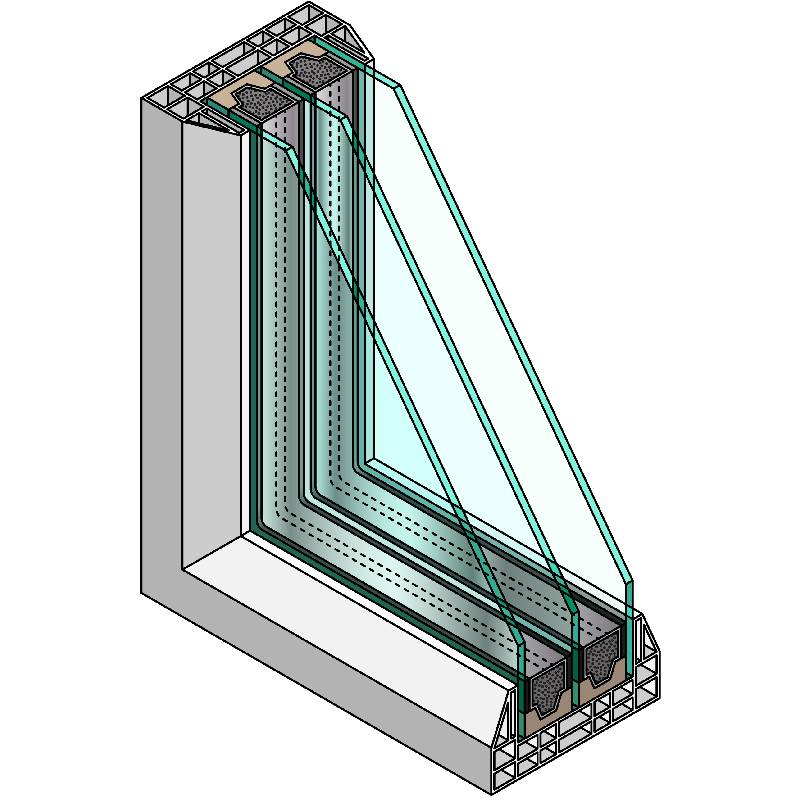
 Highly refined to minimize distortion, this type of glass is essential for lenses, prisms, mirrors, and other precision instruments where clarity and accuracy are paramount Highly refined to minimize distortion, this type of glass is essential for lenses, prisms, mirrors, and other precision instruments where clarity and accuracy are paramount
Highly refined to minimize distortion, this type of glass is essential for lenses, prisms, mirrors, and other precision instruments where clarity and accuracy are paramount Highly refined to minimize distortion, this type of glass is essential for lenses, prisms, mirrors, and other precision instruments where clarity and accuracy are paramount

 Some suppliers offer package deals that include installation, which can simplify the process and sometimes lead to overall cost savings Some suppliers offer package deals that include installation, which can simplify the process and sometimes lead to overall cost savings
Some suppliers offer package deals that include installation, which can simplify the process and sometimes lead to overall cost savings Some suppliers offer package deals that include installation, which can simplify the process and sometimes lead to overall cost savings