pharmaceutical intermediates manufacturer
-
Moreover, real-time stability studies are conducted under recommended storage conditions. These long-term studies are essential as they help to validate the findings from accelerated studies, providing a comprehensive understanding of how the API will function during its intended shelf life. The data obtained from these stability tests inform pharmaceutical manufacturers about necessary measures for handling, packaging, and labeling, ensuring that the needs of regulatory agencies, such as the FDA or EMA, are met.
...
-
Penicillin, a pioneer among antibiotics, once had a production process that caused significant environmental pollution. In recent years, with the application of eco-friendly pharma intermediates, penicillin production has become cleaner and more efficient. For instance, using biocatalysis instead of chemical catalysis not only increases penicillin yield but also significantly reduces wastewater and gas emissions, achieving green production processes. Additionally, optimizing fermentation techniques has improved the biosynthesis efficiency of penicillin, reduced chemical synthesis steps, and lowered energy and resource consumption.
...


 The reduced bulk also contributes to weight savings, which can significantly impact fuel efficiency in vehicles and the overall energy consumption in industrial machinery The reduced bulk also contributes to weight savings, which can significantly impact fuel efficiency in vehicles and the overall energy consumption in industrial machinery
The reduced bulk also contributes to weight savings, which can significantly impact fuel efficiency in vehicles and the overall energy consumption in industrial machinery The reduced bulk also contributes to weight savings, which can significantly impact fuel efficiency in vehicles and the overall energy consumption in industrial machinery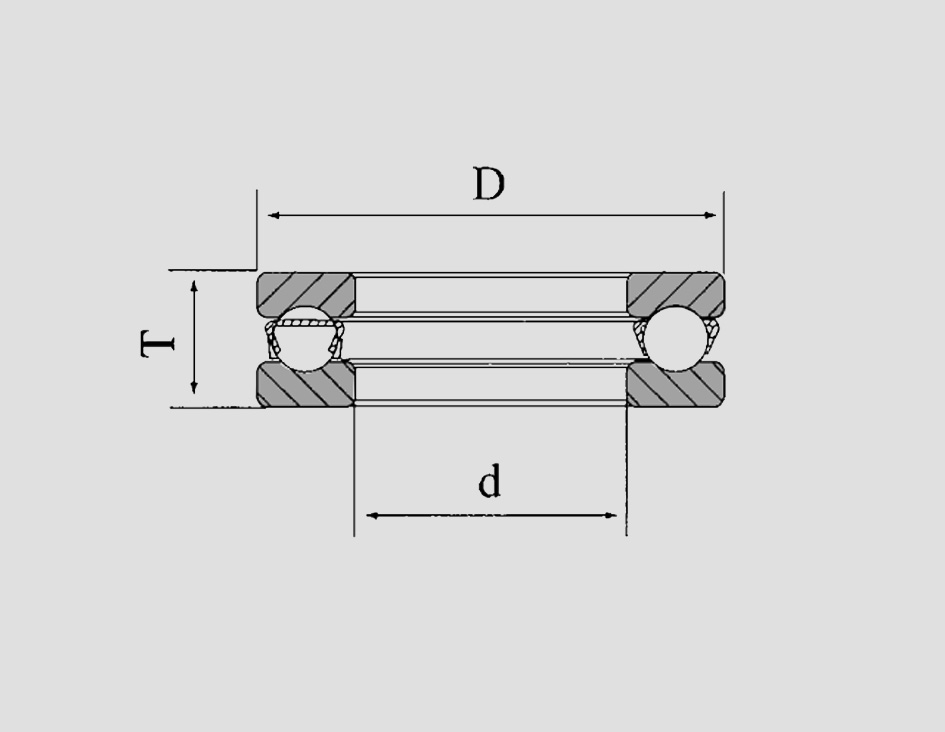
 If the bore size is too large or too small, it can cause excessive wear or damage to the bearing If the bore size is too large or too small, it can cause excessive wear or damage to the bearing
If the bore size is too large or too small, it can cause excessive wear or damage to the bearing If the bore size is too large or too small, it can cause excessive wear or damage to the bearing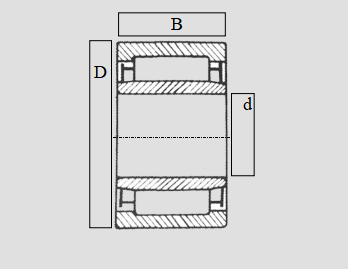 It emphasizes the importance of proper handling and lubrication to maximize bearing performance and longevity It emphasizes the importance of proper handling and lubrication to maximize bearing performance and longevity
It emphasizes the importance of proper handling and lubrication to maximize bearing performance and longevity It emphasizes the importance of proper handling and lubrication to maximize bearing performance and longevity Preloading eliminates any clearance between the balls and races, enhancing stability and rigidity, and reducing vibration and noise at high speeds Preloading eliminates any clearance between the balls and races, enhancing stability and rigidity, and reducing vibration and noise at high speeds
Preloading eliminates any clearance between the balls and races, enhancing stability and rigidity, and reducing vibration and noise at high speeds Preloading eliminates any clearance between the balls and races, enhancing stability and rigidity, and reducing vibration and noise at high speeds

 The tapered design of the rollers allows for smooth and precise rotation, making them ideal for applications that require accurate positioning and alignment The tapered design of the rollers allows for smooth and precise rotation, making them ideal for applications that require accurate positioning and alignment
The tapered design of the rollers allows for smooth and precise rotation, making them ideal for applications that require accurate positioning and alignment The tapered design of the rollers allows for smooth and precise rotation, making them ideal for applications that require accurate positioning and alignment