Once the surgical procedure is complete, sevoflurane is discontinued, and the patient begins to recover. The elimination of sevoflurane from the body occurs primarily through exhalation. The patient continues to breathe out the residual sevoflurane until the concentration in the bloodstream reaches a safe level for awakening. This process typically leads to a relatively rapid and smooth recovery.
Quality assurance is critical in API production, as impurities or variations in the manufacturing process can lead to ineffective or harmful medications. As such, companies are increasingly investing in advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and mass spectrometry, to ensure the purity and potency of their products.
Digestive enzymes are biological catalysts that facilitate the breakdown of food substances into smaller, absorbable components in the human body. They play a vital role in the digestive process, ensuring that nutrients are adequately processed and assimilated. The human digestive system produces a variety of enzymes, but three main digestive enzymes stand out as essential for efficient digestion amylase, protease, and lipase.
In conclusion, PQQ presents a range of impressive health benefits, from antioxidant protection and improved mitochondrial function to enhanced cognitive performance and cardiovascular support. Dr. Axe and others in the wellness community advocate for PQQ as a potentially transformative supplement for those looking to optimize their health. As research continues to unfold, PQQ may become an increasingly vital component in the quest for better health and longevity.
The process of developing an API typically involves several stages, including discovery, preclinical testing, and clinical trials. Once an API is developed and approved, it must be manufactured under stringent conditions in facilities that adhere to Good Manufacturing Practice (GMP) guidelines. Manufacturers must ensure that their APIs meet defined specifications for purity, potency, and consistency.
 Their durability withstands harsh weather conditions, heavy usage, and even accidental impacts, thus reducing maintenance costs over time Their durability withstands harsh weather conditions, heavy usage, and even accidental impacts, thus reducing maintenance costs over time
Their durability withstands harsh weather conditions, heavy usage, and even accidental impacts, thus reducing maintenance costs over time Their durability withstands harsh weather conditions, heavy usage, and even accidental impacts, thus reducing maintenance costs over time

 The interlocking wire mesh creates a formidable barrier that deters intruders while still allowing for easy visibility The interlocking wire mesh creates a formidable barrier that deters intruders while still allowing for easy visibility
The interlocking wire mesh creates a formidable barrier that deters intruders while still allowing for easy visibility The interlocking wire mesh creates a formidable barrier that deters intruders while still allowing for easy visibility
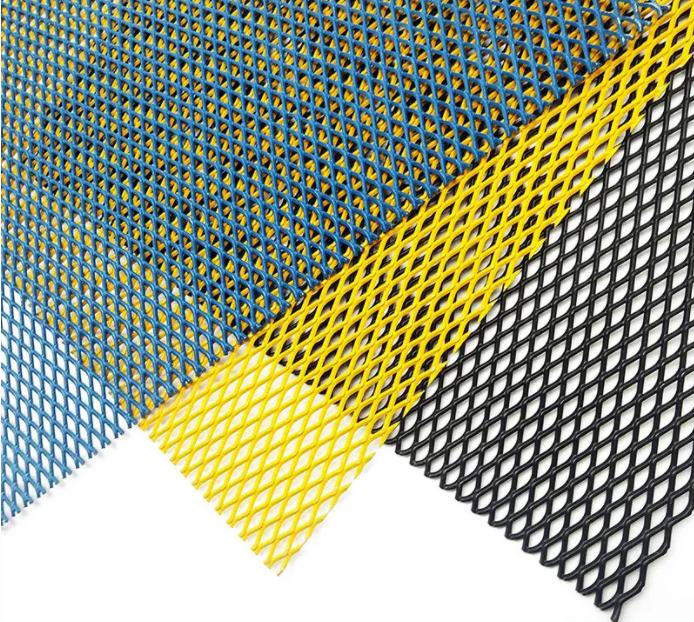 The closely packed 19 wires distribute stress evenly, reducing the risk of premature failure due to cyclic loading The closely packed 19 wires distribute stress evenly, reducing the risk of premature failure due to cyclic loading
The closely packed 19 wires distribute stress evenly, reducing the risk of premature failure due to cyclic loading The closely packed 19 wires distribute stress evenly, reducing the risk of premature failure due to cyclic loading Make sure to check that the net is securely attached to the window frame and that there are no gaps where insects could potentially enter Make sure to check that the net is securely attached to the window frame and that there are no gaps where insects could potentially enter
Make sure to check that the net is securely attached to the window frame and that there are no gaps where insects could potentially enter Make sure to check that the net is securely attached to the window frame and that there are no gaps where insects could potentially enter